During the formation of quartz crystals, some elements will replace silicon. This forms the structural impurities of quartz. The content of these impurities is very low. It is difficult to separate them from the quartz. They are the most critical factor that restricts the quality of high-purity quartz.
In the quartz structural impurities, Al impurity element content is generally the highest. Since Al exists in the form of Al3+ instead of Si4+, which causes charge imbalance within the quartz lattice, when a large amount of Al impurity exists in quartz, the content of Li, K, Na and other impurity elements will increase. Therefore, the content of Al in natural quartz can be used to judge the quality of the raw quartz material.
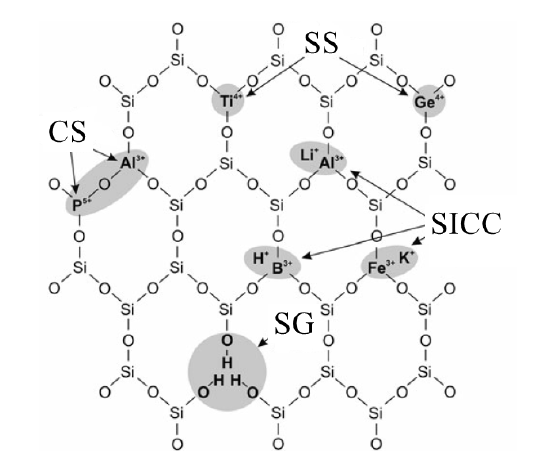
Under the existing processing technology, lattice impurities in the quartz raw material can hardly be removed. Although the content of Al element in the form of lattice impurities is very low, it is extremely difficult to remove, which is the key to the final quality of high-purity quartz.
In the whole process of purification, after roasting and quenching, magnetic separation, acid leaching process, quartz impurity elements Fe, Cr, Ni, Na, K, Ca, Mg, Cu, etc. can be greatly reduced. However, after a series of purification processes, the removal effect of Al is limited, mainly because Al3+ enters the crystal lattice to replace Si4+, and the ionic radius is relatively close to that of the quartz, which is not easy to purify.
Similarly, Ti4+, B3+, P3+ and other impurity elements. It can be seen that the impurities within the natural quartz, especially the presence of impurities in the state of homogeneous-like, directly restrict the production of high-purity quartz products when the original ore Al, Ti, Li, B, P and other impurity elements in high content, it is not easy to obtain high-purity quartz.
Typical examples are the quartzites of the Norway area, which, although co-associated with a variety of minerals such as blueschist, have few lattice impurities and contain few fluid inclusions. They are considered to have the potential to be processed into high-purity quartz, and pegmatites of the Sierra de Comechigones (Argentina) area, which, despite their high SiO2 content, are very difficult to be processed into high-purity quartz because of the high level of lattice impurities in the finer-grained quartz.
Lattice impurities, the most critical factor limiting the quality of high-purity quartz
Lattice impurities in the (Al3+, Ti4+)-O bond energy is larger, Al, Ti instead of Si in the quartz lattice to form a new [AlO4], [TiO4], is the most difficult to get rid of quartz lattice impurity elements.
Lattice structure impurities are the ultimate bottleneck of processing high-purity quartz products that are difficult to break through. To select the correct high-purity quartz raw materials and develop the best quartz purification programme, it is necessary to clarify the state of impurity elements in quartz.
The current study of impurity elements in the state of the main methods:
(1) X-ray diffraction data fitting refinement analysis;
(2) LA-ICP-MS surface scan image analysis;
(3) Infrared absorption spectroscopy analysis;
(4) Electron probe and energy spectrum analysis;
(5) Cathodoluminescence characterisation.
At present, the chlorine roasting process is a more effective way to remove impurities from the quartz crystal lattice. Chlorination roasting is below the melting temperature of quartz, and quartz impurity components and chlorine agents play a role in chloride and volatile quartz in the high-temperature chlorination roasting process. There is a crystalline transition so that the quartz lattice metal ions, such as Al3+, Ti4+, etc., may migrate and diffuse to the surface of the quartz. Hcl, NH4Cl, and Cl2, etc., to become volatile components to achieve separation of the quartz, but also to prevent impurity elements to migrate and diffuse to the quartz lattice cooling process, and then to achieve a more effective method of removal of impurities. Separation of quartz also prevents the impurity elements in the cooling process and then migrates and diffuses into the quartz lattice.

